Today’s edition of Device Talk, from Medical Device & Diagnostic Industry, describes some of the challenges facing device manufacturers contemplating the production of implantables through additive manufacturing processes. The questions raised in the article are appropriate, as no one would condone use of an additive manufactured implant if the manufacturer could not validate that the processes used resulted in material properties that enable useful life and performance of the device.
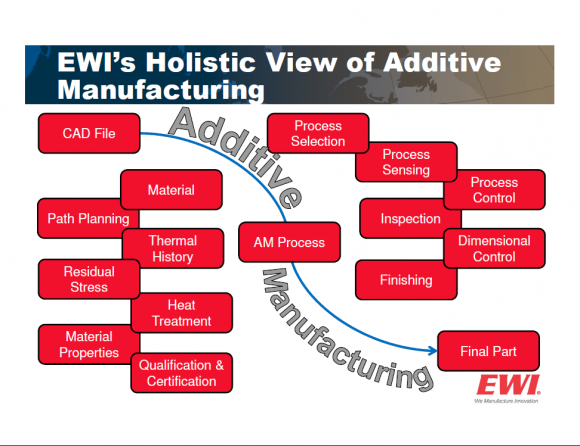
We at EWI are focused on helping manufacturers in the medical device industry characterize and define the parameters required to produce the desired material properties, and, of course, the resulting device performance. Unfortunately, many potential users of additive processes are enamored with the concept of “3-D Printing.” We wish it were as simple and straightforward as downloading a CAD file, pushing a button, and extracting the desired component. There are a lot of factors to consider when producing “3-D printed” components – factors that are critical to understand if you wish to manufacture parts that actually work as planned!
EWI’s Dr. Shawn Kelly leads our efforts to help manufacturers better define these important materials issues. His approach is shown below:
If you are contemplating an additive manufacturing process and would like to make sure the process you specify can produce the parts you need, we would be happy to help you develop and define that process. You may start by contacting Dale Robinson by phone at 614.688.5232 or e-mail at [email protected].